Optimizing immune response may improve cancer treatments
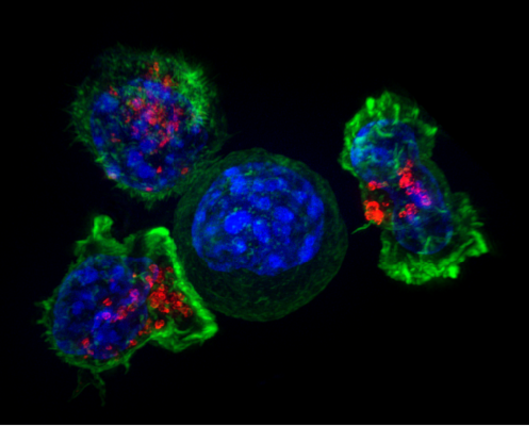
Super-resolution image of a group of killer T cells (green and red) surrounding a cancer cell (blue, center). The killer cells use special chemicals housed in vesicles (red) to deliver the killing blow. This event has been nicknamed “the kiss of death.” (Image by Alex Ritter, Jennifer Lippincott Schwartz, and Gillian Griffiths, National Institutes of Health)
Many patients undergoing cancer treatments have a problem that keeps their immune systems up at night: finding the cancer cell that got away. It’s a diabolical needle in the haystack.
“If a cell . . . was the size of a Good & Plenty candy, you could fill . . . a thousand football stadiums with the cells in your own body,” Douglas R. Green, an immunologist at St. Jude Children’s Research Hospital, told attendees at a recent conference. “And if just one of those cells becomes a cancer, we say you have cancer.”
Green spoke on Oct. 22 during the New Horizons in Science briefings produced by the Council for the Advancement of Science Writing as part of the ScienceWriters2022 conference in Memphis, Tenn.
A cancer cell multiplies through division in a deadly sort of math. If the rate of cell division is greater than cell death, tumors grow. If cell growth is less than cell death, tumors shrink. Traditional treatments such as radiation and chemotherapy give cancer cells the shove they need to self-destruct. At least, Green told the science writers, that’s the best-case scenario.
But traditional treatments don’t always kill every cancer cell. Until recently scientists thought this was because they weren’t using enough chemotherapy drugs. However, Green’s recent research, published in the journal Cell, suggests that cancer cells that survive chemotherapy share traits with so-called “persister cells.” Persister cells become tougher after stress, like trees whose roots grow deeper following a windstorm.
“Maybe we can use this knowledge to prevent [relapse] by ‘drugging’ the integrated stress response,” Green said. “Those experiments are going on now.”
The success of cancer treatment also depends on a patient’s immune response. When a cancer cell is terminated, it releases a mess of proteins, some of which are molecular flavors unique to the cancer. These are packaged and sent to the lymph nodes, where the body’s killer T cells sample the buffet of proteins. Every killer T cell is studded with receptors, each one recognizing a unique protein. When a receptor tastes a cancer-mutated protein, its parent T cell begins making copies of itself to dispose of the rest of the cancer. Scientists can optimize this response by engineering T cells and introducing them to patients.
“And very often it works,” said Green. “But not always.” The trick is convincing the T cells to remember the cancer-flavored proteins and keep making more of themselves until every needle is cleared from the haystack. One way to encourage this is to use the gene-editing system CRISPR-Cas9 or inhibitory drugs to prevent DNA from being wrapped too tightly in T cell nuclei so that the right genes stay switched on. This effectively boosts the cell’s ability to recognize the target cancer.
“If we activate T cells in the presence of this inhibitor and then wash it away, the T cells work a lot better to control cancer,” Green said.
While these recent advances are promising, the entire process takes time and a lot of money. It takes six weeks to sequence a human cancer, 48 hours to identify and engineer the right T-cell receptors, and eight more weeks to put them into a patient’s T cells and then treat the patient with a drug to ramp up T-cell production. A single treatment can cost more than $1 million.
“This is slow and it’s still a little bit theoretical,” Green acknowledged. “But it’s all going to get faster . . . because now it’s an engineering problem, and we’re really good at those.” In the future, clinics may bank T-cell receptors tailored to specific cancers the same way we currently bank specific blood types.
“We can do this,” Green said. “But we need everyone. And that’s how we’re going to cure cancer.”