Science or science fiction? The still-open questions about the #CRISPRtwins story
He Jiankui speaking at the Hong Kong International Summit on Human Genetics in November 2018. (Photo courtesy of William Kearney.)
A remarkable 2018 claim challenged both scientists and journalists.
Kiran Musunuru was shocked. In a few days, on Nov. 27, 2018, scientists from all over the world would meet in Hong Kong to set standards for the use of the CRISPR gene-editing tool on human embryos. Yet the paper in front of him suggested that in China, gene-edited twins were already growing in their mother’s uterus, with the help of scientist He Jiankui.
“I was horrified,” Musunuru recalled as he spoke to science writers gathered in State College, Pa. 11 months later, for the ScienceWriters2019 conference. “This is an historic event, the first gene-edited babies. And this is a horror show.”
That day, Associated Press reporter Marilynn Marchione had requested the opinion of three experts in genetics on an unpublished paper. Musunuru, an associate professor at the University of Pennsylvania School of Medicine, was one of them. The claims made by He, the paper’s lead author, were grandiose and terrifying: he had implanted gene-edited embryos.
He’s paper lacked peer review to establish its scientific legitimacy. Marchione thought the claim might be a publicity stunt, a show orchestrated to attract media attention. Jonathan Fahey, Marchione’s editor at AP, recalled that “we were worried about a hoax until very late in this game.”
Fahey was among the journalists attending an Oct. 28 session where Marchione, MIT Technology Review reporter Antonio Regalado, and Musunuru reflected on the remarkable #CRISPRtwins story. The session was part of the Council for the Advancement of Science Writing’s New Horizons in Science program at the science writers’ conference.
The evidence: messy and alarming
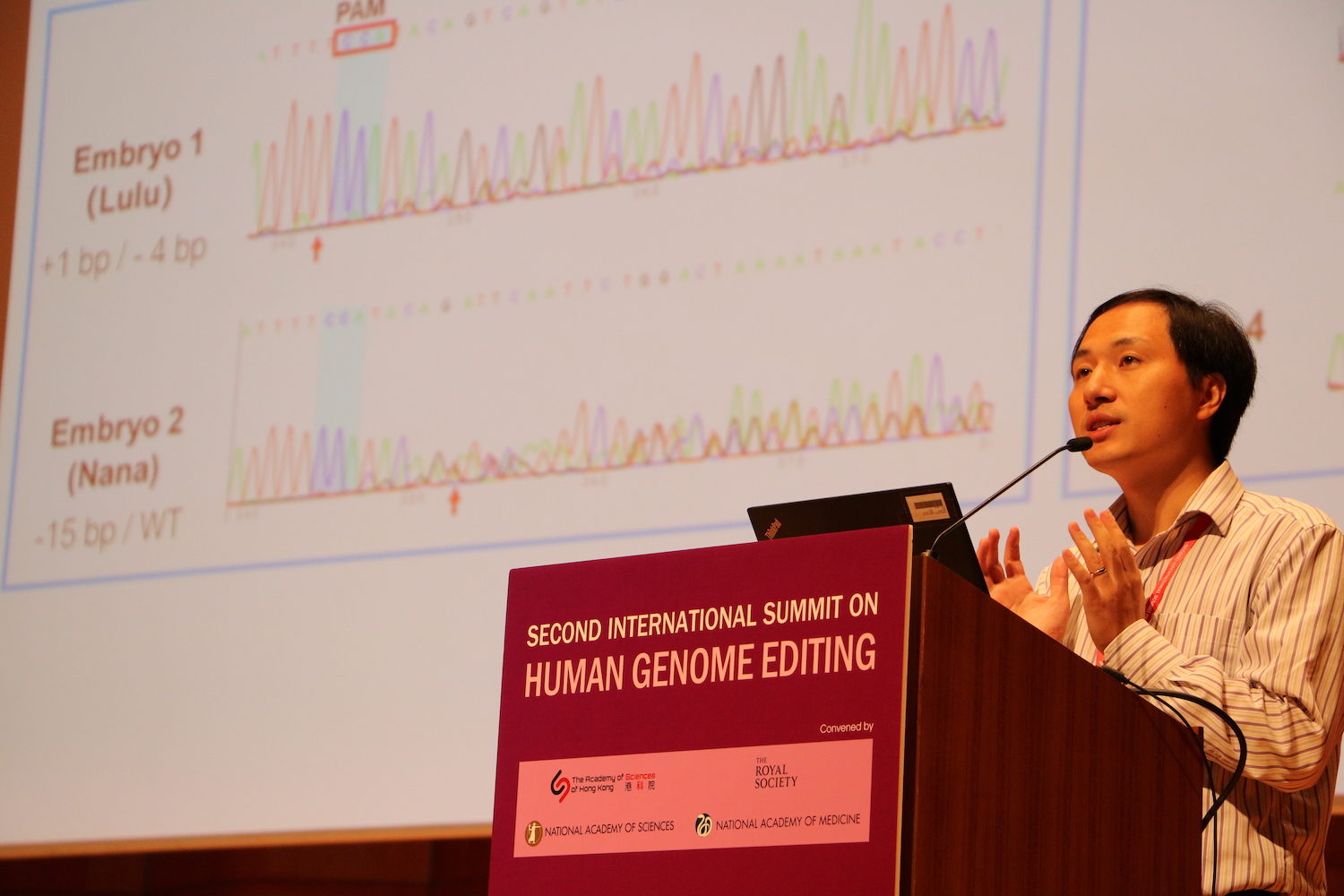
Musunuru was less doubtful. There was no question in his mind that He Jiankui had used the CRISPR-cas9 technology to suppress a gene in the embryos. “Why am I so sure that this is 100% for real? Because if you were faking this, I don’t have an explanation for why you would show such screwed-up data.”
Major flaws stood out in the data, he recalled. The CRISPR-cas9 technology had been used to scan the DNA of two embryos, search for the gene CCR5 and cut it out in order to render the babies resistant to HIV. The HIV virus uses the protein made by CCR5 as a gateway into cells, so that suppressing CCR5 protects cells from HIV. The CRISPR twins, Lulu and Nana, would have been immune from the virus had both copies of the CCR5 gene been turned off in every embryotic cell.
But some cells did not present the HIV resistance mutation. Embryo cells divide very fast, and CRISPR might not be able to edit the genes quickly enough. CRISPR “might edit some cells but not others, and you have this mix of cells with different edits,” Musunuru explained. The presence of cells that still express CCR5 would make Lulu and Nana vulnerable to HIV despite the procedure.
Musunuru also noticed evidence of off-target mutations, edits in genes that were not meant to be modified. Most likely, he said, scientists will never know the effects on the twins of these mutations, which could be passed on to future generations. He added that some changes were showing up in the data in a hit-or-miss or “mosaic” fashion, which can pose special health risks.
Heritable off-target mutations and non-medical uses of genome editing were among the ethical challenges scientists were preparing to discuss at the Hong Kong International Summit on Human Genetics as the story was developing. Gene editing has the potential to alleviate genetic diseases such as cystic fibrosis, sickle cell anemia, or Huntington disease. But it can also have unintended consequences that can be difficult to forecast.
The development of CRISPR tools has magnified those concerns. CRISPR-cas9 is cheaper and easier to use than previous generation gene-editing technologies. Musunuru compared CRISPR to fire: “If you know what you are doing, if you control it, you can get beneficial things to happen. But if you do not know what you are doing, it can spread. It can cause genetic vandalism.” With wider adoption, chances of misuse increase.
To avoid a rush to uncontrolled human applications that could backfire and damage the field, in 2015 the scientific community declared that it was unacceptable to use CRISPR on embryos and germline cells, the cells that give rise to eggs and sperm.
The objective was to maintain active exploration of gene editing and its potential to eradicate genetic diseases while minimizing unintended consequences, especially edits that could be passed on to future generations.
Struggling to report on confusing, unverifiable claims
In reporting on He’s claims, Marchione recalled that the evidence was obscure. She was able to question the research team after He’s public relations specialist, Ryan Ferrell, organized interviews. Yet she was prevented from speaking with the parents of the babies, and there had been no peer review of the documentation of the clinical trials.
In an effort to vet the science, she organized her own peer review by sharing He’s manuscript with Musunuru and two other experts in the field. Meanwhile she asked that He and his team not share their findings with other reporters. Exclusive access, Marchione said, would allow AP to investigate “without competitive pressure to publish before we were ready.” That turned out not to be possible.
On Nov. 25, 2018, two days before the Hong Kong conference was to open, Regalado broke the story.
Regalado had researched the story independently and had also been offered interviews by Ferrell. He Jiankui did not mention to Regalado that there were pregnancies under way, but Regalado was able to find documentation of the clinical trials on the internet. “We concluded [from the online documents] that there was this trial under way to make children immune to HIV,” he recalled. “We concluded that there were data about pregnancies that were six or seven months along.” Regalado reported these conclusions. Within a few hours, Marchione published her account of the claimed birth of twins with edited DNA on AP. Shortly afterward, He posted a YouTube video announcing the birth of the #CRISPRtwins.
Marchione and Regalado felt and still feel the reporting of the story is unfinished. “I had the sensation this might be the most important story that I have ever done—or the last story that I do” Regalado recalled.
After all, while plausible, nobody knows whether He Jiankui’s claims are true. There remains no proof that the two babies actually exist. The alleged birth of a third gene-edited baby is unverified, and He’s location is currently unknown.
Musunuru was relieved when the Tech Review and AP stories were published. “I was so grateful when I realized the news had broke.” In the wake of international condemnation, He’s experiments were stopped.