
CRISPR: Genome engineering with a purpose
Photograph of George Church by Bethany Bella.
A new technology for engineering genomes called CRISPR has implications for human aging as well as the resurrection of certain extinct species, according to Harvard Medical School scientist and engineer George M. Church, who briefed science writers Oct. 19 during CASW’s New Horizons in Science, part of the ScienceWriters2014 conference in Columbus, Ohio.
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) technology is different from most earlier gene-modifying techniques in that it can be used to change one gene, many genes or, even more radically, an entire genome. Depending on the genetic sequence being targeted, the mutations may appear in a single location or across an entire genome.
Where once scientists executed genetic manipulations with a certain degree of uncertainty, CRISPR has enabled precise genome-cutting techniques.
An organism’s genome—its complete set of DNA—contains all of the necessary biological information for life. Many genes—stretches of DNA—code for specific proteins, the workhorses that carry out the processes required for life. Non-coding parts of the genome stop, start, promote or suppress protein-coding genes, regulating how they are expressed in specific cells over the course of a lifetime.
Often genetic information is not copied correctly, and mutations arise along the DNA sequence, causing a range from minor defects to potentially life-threatening genetic diseases.
In 2012, Jennifer Doudna of the University of California, Berkeley, Emmanuelle Charpentier of the Helmholtz Centre for Infection Research and Martin Jinek of the University of Zurich showed that the CRISPR system—derived from the biochemical tricks bacteria use to defend against viruses—can be used to modify the human genome by inserting a customized DNA sequence in a specific section of DNA. This “genome editing” operation takes place while the double strands of DNA are unzipped.
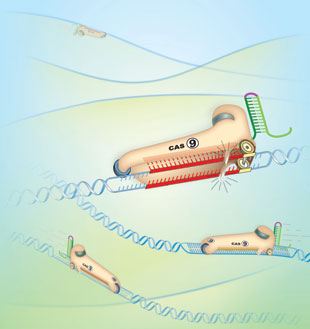 In the new technique, a deletion enzyme erases the undesired section of DNA, while the CRISPR snippet precisely guides the insertion of a modified sequence. This scissor-like technique of cutting and repairing target DNA sequences makes CRISPR extremely accurate. (The graphic at right, published by The Scientist, shows the enzyme Cas-9 snipping DNA under the guidance of CRISPR snippets.)
In the new technique, a deletion enzyme erases the undesired section of DNA, while the CRISPR snippet precisely guides the insertion of a modified sequence. This scissor-like technique of cutting and repairing target DNA sequences makes CRISPR extremely accurate. (The graphic at right, published by The Scientist, shows the enzyme Cas-9 snipping DNA under the guidance of CRISPR snippets.)
Church’s group has been aggressively exploring applications of CRISPR and advocating broad public discussion of the environmental and social implications of the technique, which is expected to make genome engineering widely available and economical.
Reversing human aging and controlling pests
In addition to providing a higher degree of accuracy and precision to genetic insertion, Church said in his Columbus presentation, CRISPR-like technologies could potentially be used to reverse the human aging process.
“We are all going to die of aging, probably,” Church predicted. “Ninety percent of people in places with good medical care are dying of aging.”
He noted that the purpose of reversing aging was not to create eternal life.
“The objective is not to extend death, to extend the worst part of our lives, but to reverse aging. Reversal of aging is much easier to do than longevity,” Church said. “We’re attacking this; my lab and others are looking at full genome sequencing of super-centenarians. It’s probably not their environment that’s making the rule on time, but hopefully rare factors.”
Pests, crops and gene drives
Church said CRISPR could also be used to alter wild populations.
“What if we wanted to change a whole population?” Church asked. “Probably not people, but plants or animals that might constitute pests; threats to human health; threats to the environment; threats to agriculture; invasive species; vectors that spread diseases like ticks, flies, and mosquitoes; and sustainable agriculture.”
The use of genetic engineering in agriculture has sparked controversy around the world as anti-GMO (genetically modified organism) activists have raised questions about the safety and environmental and economic effects of GMO crops.
In a New Horizons session following Church’s presentation, Allison A. Snow of Ohio State University’s Department of Evolution, Ecology and Organismal Biology briefly mentioned the implications of CRISPR for the science of GMOs in another New Horizons session. The genetic modification of crops, she said, is akin to “genetic engineering on steroids.”
“We heard about CRISPR technologies this morning,” Snow said. “That technique could be used to improve crop plants. There’s a lot of work going on right now to see how easily this technique could be used to replace previous techniques.”
Over the history of agriculture, important crop species such as corn, soy and potatoes have been bred to reduce threats from blights, invasive species or drought. However, a monolithic food crop culture has its own challenges, including a severe reduction in genetic diversity, and in some cases a reduction in nutritional content.
“We also heard this morning about how we could eradicate unwanted populations, like mosquitoes and rats and weeds,” Snow said. “But that’s all in the future, it’s not definite that that could happen.
“It’s good to be aware of it, and start those discussions,” she said.
Church, the founder of the Personal Genome Project, explained how CRISPR-enabled advances would allow farmers to replace either their natural or synthetic pesticides using what is known as a gene drive to block the harmful effect of a population of crop pests.
“This is an extreme form of genetics, where we’re not only putting genetics out into the wild, as we do with gene therapy and GMO crops,” Church said. “These [genome-modified pests] are out in the wild, and they’re spreading intentionally.”
A “woolly elephant”?
One additional application of CRISPR technologies includes the speculative, science-fiction reality of de-extinction, or the resurrection of extinct animals. Fortunately, or perhaps unfortunately for some, only the extinct animals whose preserved DNA humans retain can qualify.
“The past is not necessarily the future,” Church said. “It’s not just about nostalgia, it’s about making economic arguments that are good for the environment and good for the bottom line.”
The particular ancient species that Church and his colleagues are attempting to re-introduce is the woolly mammoth through genetic modification of a modern relative, the Asian elephant.
“It turns out that the mammoths are much more closely related to Asian than to African [elephants],” Church said. “In fact, they’re so close, they’re both closer to each other than they are to the African elephants.”
Speculation over the ethics and selection of such a species raises several questions for the current field of genetic engineering.
“This is not about making the perfect woolly mammoth.” Church elaborated. “This is about getting cold-resistant animals, in particular elephants.”
Genetic de-extinction requires significant DNA manipulations. “We’re making multiple mutations in multiple genes having multiple traits,” Church explained. “In the case of the elephant, we have already made 15 changes with CRISPR. We’ve changed the hemoglobin gene, genes involved in subcutaneous fats, sebaceous glands, short woolly hair, long hair and small ears.”
As Church and his genome-engineering colleagues attempt to resurrect the woolly mammoth and use CRISPR technology to modify crops, the ethical implications of such technologies remain to be assessed.
“It really is up to society to decide what is the most safe and effective,” Church said. “We’re not the greatest at preventative medicine, but this is like the quintessential preventative medicine.”
Expanded knowledge of the human genome has paved the way for an evolutionary of science in the twenty-first century—one that seems to defy what human beings can and will achieve in the future. “I’m here to report that human genetics have been working quite well,” said Church. “It’s not getting worse, it’s getting better.”
As CRISPR rapidly ripens into a more prominent technology in laboratories around the world, Church maintains a resolutely forward-looking mindset.
“I like talking about the future,” Church said. “CRISPR is not the end of the story. I am passionate about it, but I am passionate more about genome engineering—to make it more safer and more effective.”